MISSION-Fumarate Trial
Validation of hyperpolarised [1,4- 13C 2] fumarate as a candidate for prognostic and treatment response marker for RENAL CANCER
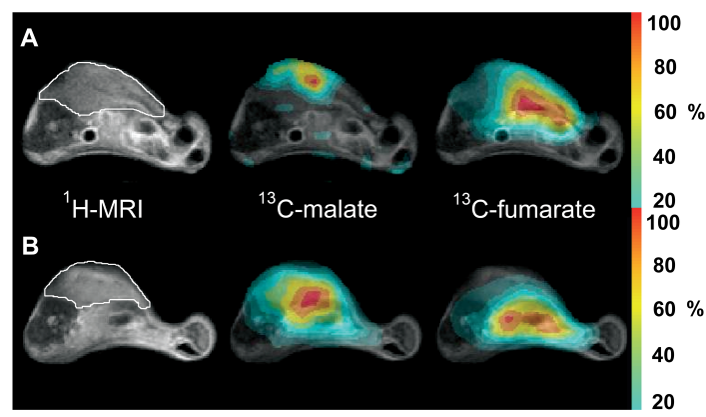
We have shown in preclinical studies that hyperpolarised [1,4- 13C 2] fumarate magnetic resonance imaging (MRI) can be used as an imaging biomarker to assess tumour necrosis.
The hyperpolarised MRI agent [1,4- 13C 2,2,3-d 2] fumarate has completed the required preclinical safety and toxicology studies and now has ethical approval for a first-in-human clinical study, led by Professor Ferdia Gallagher. Validation is now underway prior to commencement of the study.
Our Exemplar 2 study (MISSION-Fumarate study) will be conducted at 2 centres, the University of Cambridge and University College London, to evaluate hyperpolarised [1,4-
13C
2,2,3-d
2] fumarate in renal cancer patients scheduled for nephrectomy.
13C magnetic resonance spectroscopic imaging (
13C MRSI) measurements of tumour malate production as a marker of tumour cell death will be undertaken prior to surgery. Cell death will also be assessed using diffusion weighted MRI and histological examination and the results compared to those obtained using hyperpolarised [1,4-
13C
2,2,3-d
2] fumarate MRI.
The MISSION-Fumarate study (
ISRCTN49119680) is now open for recruitment. See our
MISSION Fumarate patient resources page for more details on the study.
Study Collaborators:
Prof Shonit Punwani, University College London;
Prof Grant Stewart, University of Cambridge and
Prof Ian Wilkinson, University of Cambridge.