[18F]Fluorodopa PET and Oxygen Enhanced MR Imaging in Glioma: Feasibility Study for Advanced Imaging Guided Brain Biopsy (FIG Study)
What is the purpose of this study?
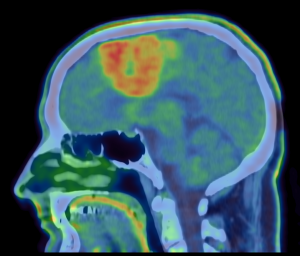
We think that [18F]Fluorodopa (FDOPA) PET and Oxygen Enhanced MRI might help determine the grade and type of glioma more accurately when used alongside standard MRI. We need to establish whether using these advanced imaging techniques is feasible and the information from the study may form the basis of further clinical research.
Why is this research being done?
The only way to know the type and grade of a brain tumour for 100% certainty is to perform a biopsy which is an invasive procedure and has a number of risks involved with it. A certain type of medical imaging called amino acid Positron Emission Tomography (PET) has been shown to be better in distinguishing between high and low grade gliomas. Alongside this, a type of novel MRI technique, called oxygen enhanced MRI (which is a MRI scan performed whilst wearing an oxygen mask), has the ability to detect areas of hypoxia (low oxygen levels) within solid tumours which would potentially detect more aggressive high-grade areas within gliomas.
What are the benefits of taking part in the study?
The imaging we are trying out in this study, called FDOPA PET and Oxygen Enhanced MRI, allows us to look at the metabolic activity within the tumour to identify areas that may be behaving differently. If the study confirms that these advanced imaging techniques is feasible we would then be able to develop larger research studies to look at whether these imaging techniques could help identify the grade and type of glioma without the need for a biopsy.
Who is being included in the study?
We are planning to enrol 21 people from across Oxford, Cambridge and Imperial College London with a new diagnosis of a glioma that are undergoing surgical resection of their tumour.
What is the status of the study?
Recruitment for the FIG study has been completed.
Who is carrying out the study?
The study is being conducted by University College London, who is the sponsor of the research. The study is funded by Cancer Research UK.
If I have any questions, who can I contact?
If you have any questions please speak to your hospital medical team. You can also contact us by email at ncita.fig@ucl.ac.uk
For more information see the FIG study record on ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04870580

