Novel and multi-parametric (mp) MRI PROSTATE image repository for development of artificial intelligence automated reporting and multi-centre clinical trials
Project Lead:
Professor Shonit Punwani (University College London)
Previously the standard practice for diagnosing prostate cancer involved a non-targeted biopsy following a raised prostate specific antigen (PSA) blood test. Multi-parametric magnetic resonance imaging (mp-MRI) now offers image-based diagnosis of prostate cancer in patients with elevated PSA levels, improving diagnostic accuracy by the use of targeted biopsies of suspicious lesions visible on mp-MRI. (Prostate MRI imaging study (PROMIS) registered on ClinicalTrials.gov as
NCT01292291, Prostate Evaluation for Clinically Important Disease: Sampling Using Image-guidance Or Not? (PRECISION) registered on ClinicalTrials.gov as
NCT02380027).
NCITA Exemplar 4, led by Professor Shonit Punwani, UCL, aims to progress the utility of mp-MRI for adoption by the NHS, through the creation of a novel mp-MRI repository and development of artificial image analysis tools. NCITA will establish an annotated prostate dataset from the PROMIS study data, including patients both positive and negative for prostate cancer, which will be hosted on the NCITA repository. This dataset offers a unique opportunity to validate artificial intelligence algorithms for detection and grading of prostate cancer to improve radiologist performance using machine learning to identify abnormal mp-MRI scans and automate the reporting of normal multiparametric MRI image scans, thereby reducing NHS costs.
A proportion of these scans will be re-reported according to Pi -RADS v2 criteria, and correlated with the corresponding histopathology results to allow comparison to the alternative Likert scoring system. The annotated data will be made available to NCITA institutions together with other parties developing machine learning focused on addressing the unmet clinical needs.
The NCITA repository will also upload datasets acquired as part of the single centre INNOVATE trial which include mp-MRI and the novel Vascular, Extracellular and Restricted Diffusion for Cytometry in Tumours (VERDICT) sequence (registered on ClinicalTrials.gov as NCT02689271) onto the NCITA repository. A processing pipeline which will be established and shared between NCITA host institutions to aid preparations for multicentre studies.
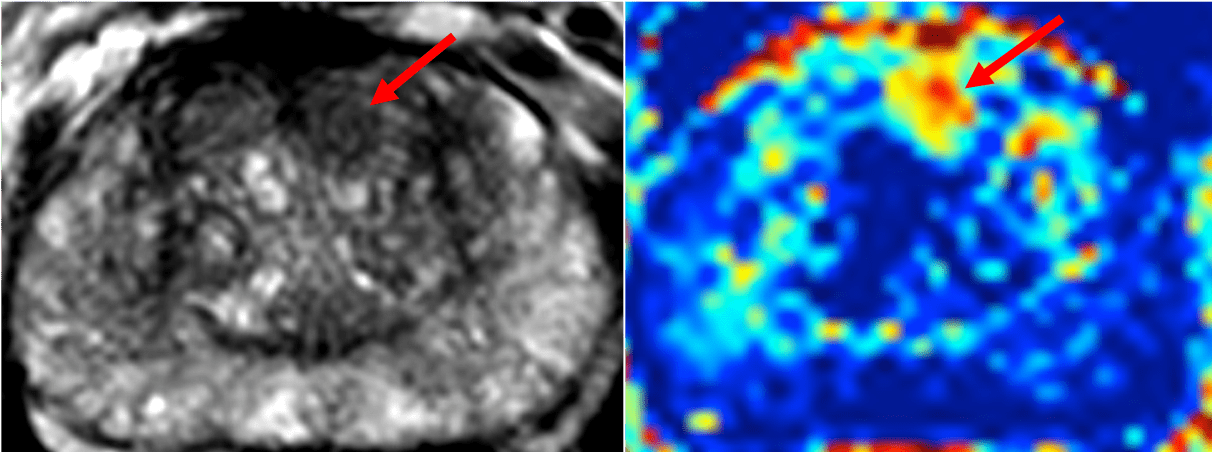
Images from the INNOVATE trial ( https://bmccancer.biomedcentral.com/articles/10.1186/s12885-016-2856-2), courtesy of Professor Shonit Punwani and Dr Saurabh Singh, University College London, Centre for Medical Imaging

