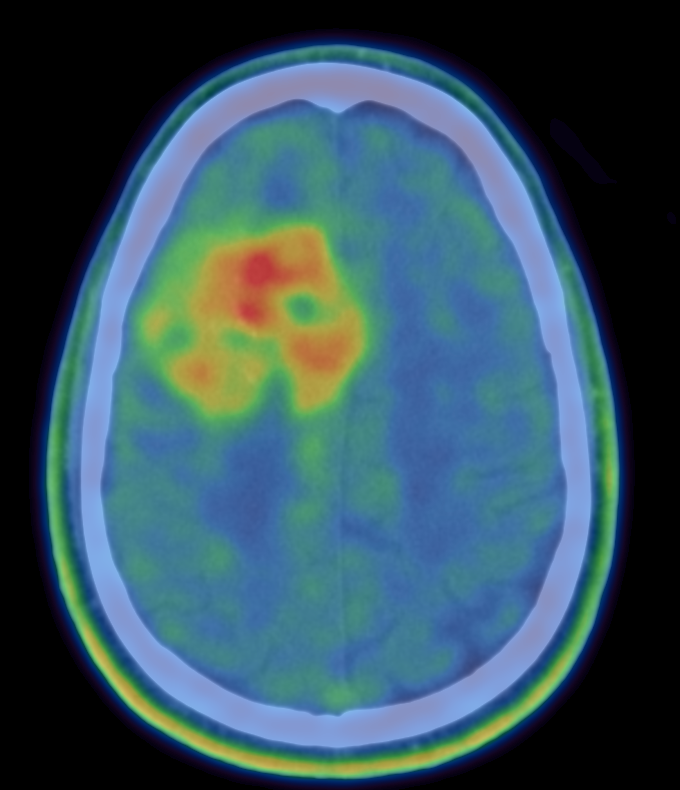
The FIG Trial
18F-FDOPA PET imaging in GLIOMA: feasibility study for PET guided brain biopsy
Project Lead: Professor Geoff Higgins, University of Oxford
The radiotracer 3,4-dihydroxy-6-[ 18F] fluoro-L-phenylalanine ( 18F-FDOPA) has been shown to be a promising imaging agent for positron emission tomography (PET) imaging evaluation of brain tumours.
The NCITA Exemplar 7 FIG study (
ClinicalTrials.gov Identifier: NCT04870580) is a multicentre phase 1 study which is assessing the feasibility of performing
18F-FDOPA PET guided histopathology using standardised PET imaging protocols. The study was set up initially at the University of Oxford, and then expanded to Imperial College London.
The FIG study also includes assessment of the feasibility of oxygen-enhanced MRI (OE-MRI) for the detection of tumour hypoxia, in collaboration with The University of Manchester and UCL.
See our
FIG study patient resources for more details about the study.
This Exemplar study will provide the feasibility imaging data required to apply for further funding to perform a multicentre clinical evaluation study using 18F-FDOPA PET guided biopsies.