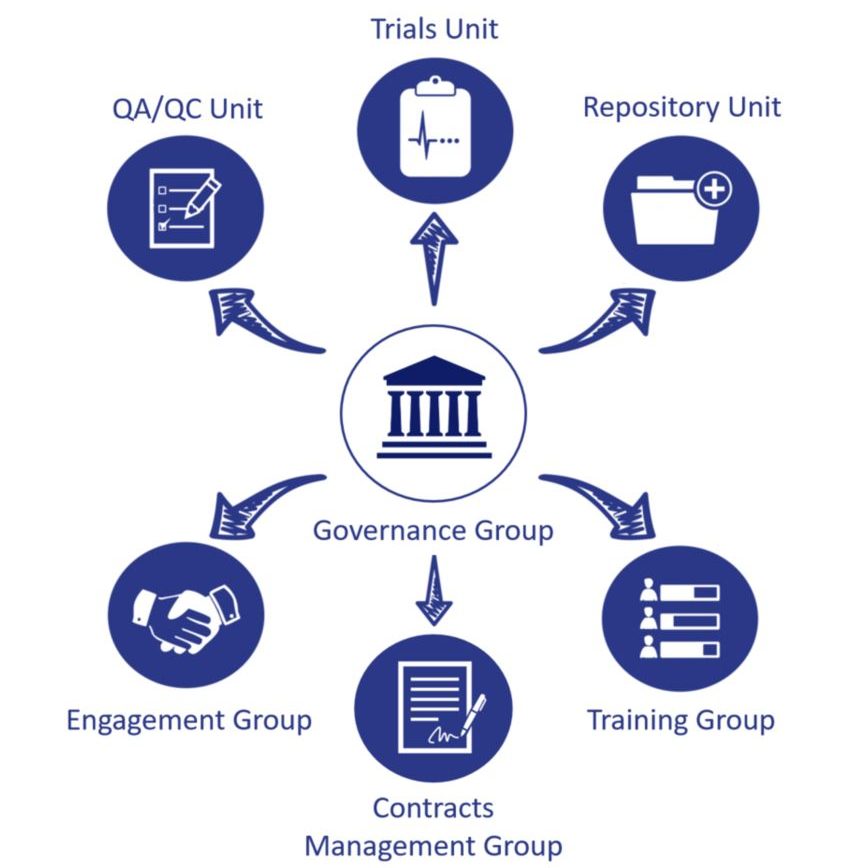
NCITA Units
The NCITA Infrastructure is composed of three cross -institutional units and three activity groups which work in synergy to provide a coordinated infrastructure and integrated pipeline for the development of quality assured cancer imaging biomarkers for clinical use.
Imaging Clinical Trials Unit

The NCITA Imaging Clinical Trials Unit (CTU) supports clinical studies, where the research question focuses on imaging, or imaging is required to determine the primary end point.
The CTU is a cross-institutional network of imaging centres across the UK, which provide bespoke trial management and governance support for clinical imaging studies using cutting-edge magnetic resonance imaging (MRI) and nuclear imaging techniques.
Working closely with the NCITA QA/QC Unit and the Repository Unit, the NCITA CTU is developing robust standardised image acquisition, processing and analysis tools for the development of imaging biomarkers, from first-in-human application andsingle-site reproducibility assessment to multi-centre clinical studies required for standardisation and potential certification of imaging biomarkers.
The NCITA CTU is directed by Professor Dow-Mu Koh (Institute of Cancer Research, London) and Professor Stuart Taylor (UCL) , and is integrated with the UCL Biomedical Research Council (BRC) Imaging Trials Unit.
Our statistical collaborator and advisor is Professor Sue Mallett , Professor in Diagnostic and Prognostic Medical Statistics, UCL, Centre for Medical Imaging.
QA/QC Unit

The NCITA Quality Assurance and Quality Control Unit (QA/QC) aims to convert imaging biomarkers from undefined lab-based metrics into locked down, quality assured imaging toolkits for clinical use .
The QA/QC unit provides robust validation, standardisation, quality assurance and quality control support for MRI and nuclear imaging biomarker studies, from first-in-human to multi-site reproducibility assessment studies.
The QA/QC unit leverages the core strengths in imaging techniques and chemistry of our NCITA partners, including The University of Manchester, University of Oxford, University of Cambridge, King’s College London, Imperial College London, University College London and The ICR, in conjunction with the NCITA CTU and Repository Unit.
Our NCITA QA/QC unit has also established a national NCITA MR Core Lab to coordinate the implementation of standardised, quality-assured procedures for MRI and magnetic resonance spectroscopy (MRS) clinical research studies. This will help elevate the quality of MRI biomarkers to a level akin to nuclear imaging biomarkers, which is a current unmet need within cancer imaging research both in the UK and worldwide.
The NCITA QA/QC Unit is directed by Professor Eric Aboagye (Imperial College London) . The NCITA MR Core Lab lead is Dr Penny Cristinacce (University of Manchester) .
Repository Unit
The NCITA Repository Unit provides an image repository and data management service for secure storage of imaging biomarker trial data and sharing of anonymised datasets between trials sites in multicentre clinical trials. The repository is based on a comprehensive platform for secure archiving, processing and sharing of research imaging data previously developed by the CRUK Cancer Imaging Centres (CIC) initiative.
The Repository Unit will develop the platform to provide a sustainable repository for storage, processing and sharing of clinical imaging trial datasets for NCITA Exemplar studies, as well as data sharing with external academic and industrial partners. Over the course of the 5-year CRUK Accelerator award, the NCITA Repository Unit will also work to develop the platform to enable data clearance for release as a community resource, making possible the linking of curated imaging datasets to publications and generation of datasets for artificial intelligence research.
The Repository Unit is directed by Professor Kevin Brindle (University of Cambridge) and managed by the Repository Unit Manager Dr Simon Doran (Institute of Cancer Research, London) .
Engagement Group

The NCITA Engagement Group facilitates the NCITA infrastructure through engagement with imaging biomarker consumers (NHS, pharma, academic institutions, patient groups) and service providers (medical imaging, nuclear medicine and medical data management companies). The Engagement Group, in conjunction with the Governance Group, are establishing a Consensus Group of key stakeholders to agree quality assurance characteristics for the validation and certification of imaging biomarkers for clinical use and adoption into the NHS; the results of which will be published in a Consensus paper.
The Engagement Group disseminates NCITA news, events and information through the NCITA website, social media, press releases and publications. The Engagement Group also facilitates new industrial, academic and clinical collaborators to access NCITA infrastructure support for clinical imaging biomarker validation studies, and Big Data research using imaging biomarker readouts.
The NCITA Engagement Group is directed by Professor Geoff Higgins (University of Oxford) and managed by the NCITA Project Manager and Engagement Coordinator Dr Martina McAteer (University of Oxford) .
Training Group

The NCITA Training Group are continuing the educational training initiated by the CRUK Cancer Imaging Centres (CICs) by training the next generation of cancer imaging scientists through the organisation of regular training events and an annual national NCITA Conference. The Training Group support the NCITA network by developing bespoke training workshops relevant to the Exemplar projects, or projects adopted by the NCITA infrastructure.
The NCITA Training Group are also developing a platform of online teaching materials for NCITA staff as well as the broader cancer imaging community.
The NCITA Training Group is directed by Professor James O’Connor (Institute of Cancer Research and University of Manchester) .
Contracts Management Group

The NCITA Contracts Management Group is responsible for managing and completing all contracts required to meet NCITA’s strategic priorities. This includes the overarching collaboration agreements between the NCITA partner institutions and Cancer Research UK, as well contracts with external commercial and academic partners for studies adopted through the NCITA study adoption process.
The Contracts Management Group is developing a template study contract for collaborating institutions to reduce start-up times for multicentre clinical studies and improve study efficiency. The group includes contract management representatives from the 7 NCITA funded partner institutions and may also include, on an ad hoc basis, external commercial and non-partner academic contract representatives as required.
The NCITA Contracts Management Group is directed by Professor Tony Ng (King’s College London & UCL Cancer Institute) and managed by the NCITA Contracts Manager Mr Philip Ryan (UCL).
NCITA Exemplar Projects
NCITA is supporting a portfolio of Exemplar projects in areas of unmet clinical need to demonstrate the effectiveness of the NCITA infrastructure in accelerating the standardisation and clinical translation of cancer imaging biomarkers.
NCITA leaders work closely with the Cancer Research Horizons team to establish the best commercial model to ensure new discoveries from the Exemplar projects and other projects supported by Cancer Research UK become available to patients with cancer.
Investigation of short-chain fatty acid uptake in solid tumours by [
18F] fluoropivalate (FPIA) PET and its relationship with tumour proliferation in GLIOMA and other cancers
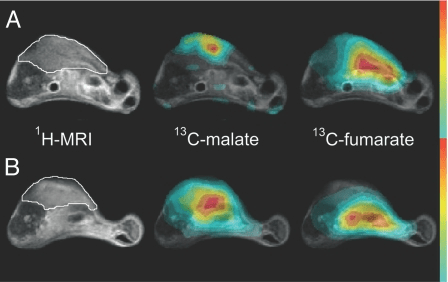
Validation of hyperpolarised [1,4-
13C
2,2,3-d
2] fumarate as a candidate for prognostic and treatment response marker for RENAL cancer
Establishing the environment for UK multicentre clinical evaluation of whole-body (WB) MRI as a diagnostic and treatment response marker in MULTIPLE MYELOMA
Novel and multi-parametric (mp) MRI PROSTATE image repository for development of artificial intelligence automated reporting and multi-centre clinical trial
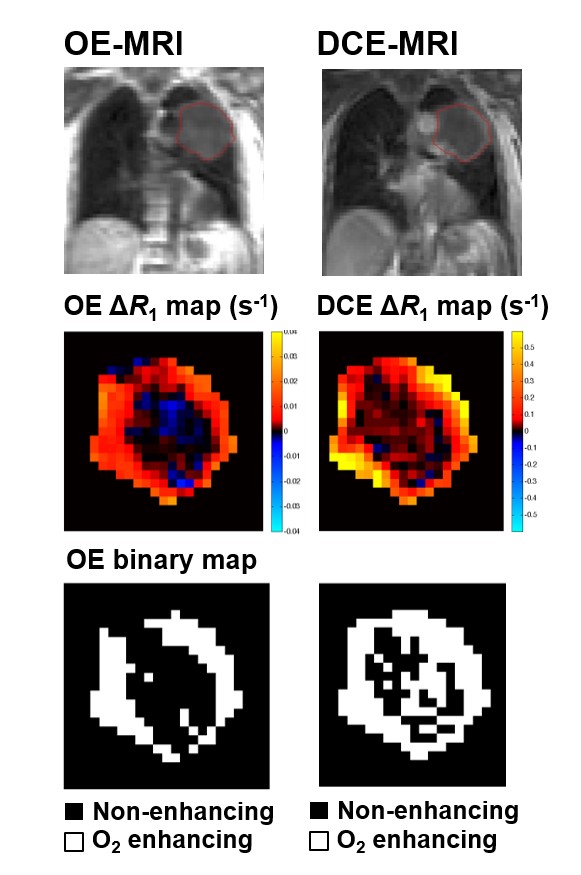
Oxygen enhanced magnetic resonance imaging for patients with lung cancer receiving chemoradiation
Exosome analysis of HER2 expression and heterodimerisation in patients from the HERPET study at Imperial College London
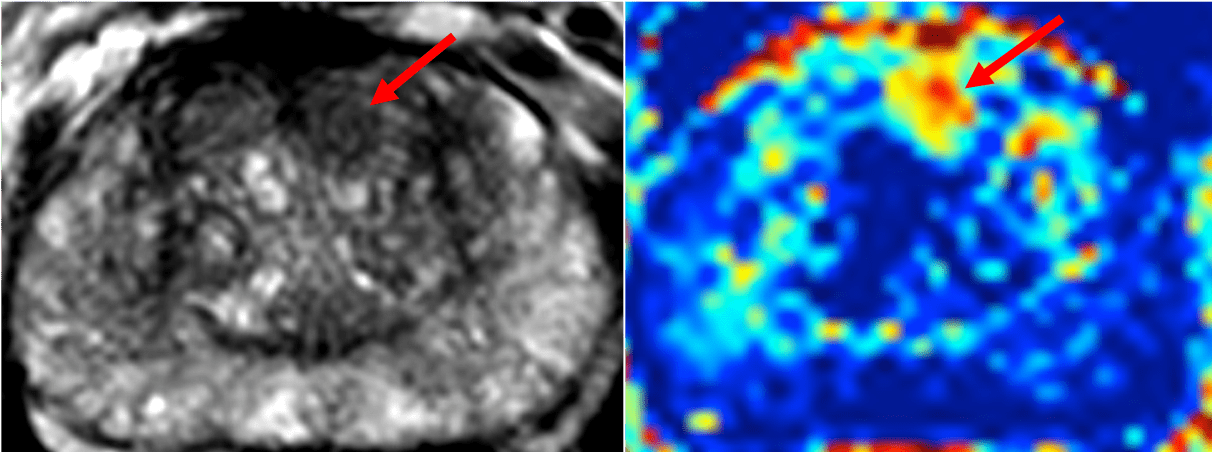
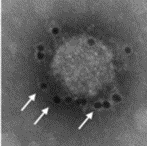
18F-FDOPA PET imaging in GLIOMA: feasibility study for PET guided brain biopsy
Adoption of Clinical Imaging Research Studies
NCITA is collaborating with clincial researchers across the UK to provide NCITA Repository, Imaging Clinical Trials Unit and QA/QC Unit support for multicentre clinical imaging biomarker studies.
NCITA infrastructure support is available to external partners from both academia and industry, provided the clinical research proposal meets the NCITA study eligibility criteria.
For more information on our study application process, please contact us at ncita.general@ucl.ac.uk or using our contact form.
External Studies Adopted by the NCITA Infrastructure
Atovaquone with Radical ChemorADiotherapy in Locally Advanced NSCLC
NCITA Repository Unit
Plasma Analysis for Response Assessment and to Direct the manaGement of Metastatic prostate cancer imaging sub-study
NCITA CTU, QA/QC Unit, Repository Unit
International Alliance for Cancer Early Detection Alliance (ACED)
NCITA Repository Unit
LIBRA
Lung Nodule Imaging Biobank for Radiomics and AI Research
NCITA Repository Unit
CLIMATE
Comparison of diagnostic ccuracy of Luminal Index and Multi-parametric MRI for Accelerated deTEction of significant prostate cancer
NCITA CTU, QA/QC Unit, Repository Unit
LIMIT
Luminal Index MRI Identification of Treatment critical Prostate Cancer (LIMIT PCa)
NCITA CTU, QA/QC Unit, Repository Unit
HERD
Multimodality Early Detection of Head and Neck Cancer Recurrence
NCITA CTU, QA/QC Unit, Repository Unit
Breast Screening – Risk Adaptive Imaging for Density (BRAID)
NCITA Repository Unit
Integrated Image Analysis in High Grade Serous Ovarian Cancer
NCITA Repository Unit
Lymphatic Mapping Of Oropharyngeal Cancer (LOOC)
NCITA CTU, Repository Unit
PANTHR-S
Precision medicine Approaches for Neoadjuvant Therapy in high‐Risk Sarcoma patients
NCITA Repository Unit
LOCATE
Investigating the application of WB-MRI for re-staging of prostate cancer patients with biochemical relapse (BCR) following external beam radiotherapy and brachytherapy
NCITA Repository Unit
Re-IMAGINE Prostate Cancer Screening - Inviting Men for Prostate Cancer Screening using MRI
NCITA CTU, Repository Unit
Novel methodologies for the early detection of hepatocellular carcinoma
NCITA Repository Unit
VALIDATE-PRO
Novel MRI assessment of prostate cancer
NCITA CTU, Repository Unit
BeSPOKE
Building and Evaluating a risk Stratified PrOstate pathway for cancer screening, detection, treatment and surveillancE
NCITA CTU, Repository Unit
STARTER-KIT
Development of a technical, procedural and ethical template for accelerating the start-up of AI multicentre studies requiring data re-use and/or sharing in clinical settings
NCITA Repository Unit


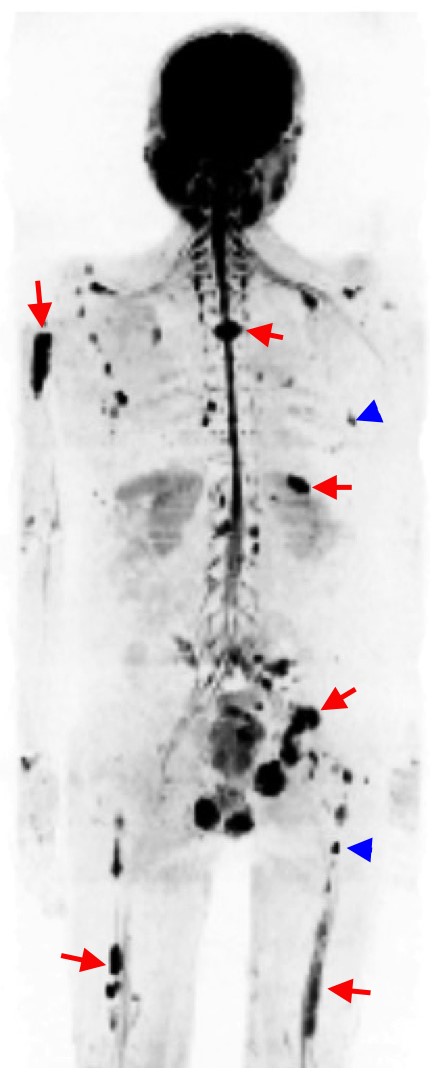
![Exemplar 1 image - [18F]FPIA maximum intensity projection (MIP) image showing normal biodistribution in a healthy volunteer. Note minimal background activity aside from liver.](../wp-content/uploads/2020/02/Figure-1-exemplar-1-2.png)